Back to Alexey Onufriev's home page
PROTON-PUMPING MECHANISM
Energy diagram
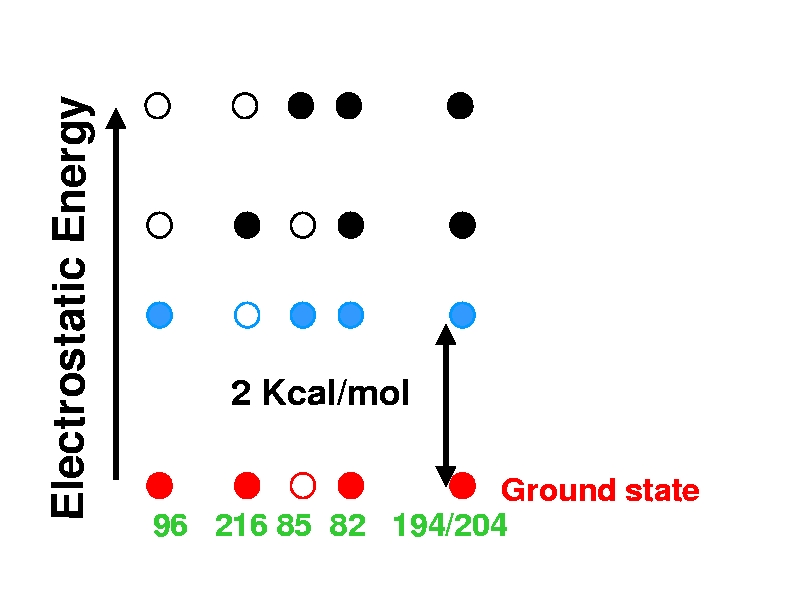
Four lowest protonation states of wild-type bacteriorhodopsin
at pH=7. Filled and empty circles represent protonated and de-protonated
sites respectively. The calculations are based on a structure representing the
BR (resting) state of the photocycle. In the ground protonation
state, Asp96 is protonated,
Schiff Base (216) is protonated, Asp85 is deprotonated, and Arg82 is
protonated. The release group is protonated, sharing a proton
between residues Glu194 and Glu204. A large, ~ 3kT, gap between
the ground and the first excited protonation states ensures that the ground
state is realized at room temperature and in a wide range of pH. This gap is
the basis of experimentally observed extreme robustness of the BR molecular
machine which, in Nature, functions efficiently under very broad range of ambient conditions.
